Artificial cochlea for natural hearing
People with hearing deficiency are greatly disadvantaged in a world in which so much is carried by the spoken word. The neuroinformatics scientist Ruedi Stoop and his team from the Institute of Neuroinformatics of Zurich University and ETH Zurich have now developed a mathematical model that has enabled them to build an electronic copy of the cochlea.

Two rows of interconnected circuit boards with countless capacitors, resistors, transistors and chips lie on a table in Ruedi Stoop’s cramped office. Coloured cables protrude from the tangle, and behind it is a stack of electronic instruments that generate electrical signals. The professor of Neuroinformatics at the Institute of the same name at ETH Zurich and the Zurich University says the equipment functions in exactly the same way as a human cochlea (inner ear): “In principle this model is a replica of an unrolled cochlea.”
Replicating the Cochlea is no easy task, because hearing is one of the human being’s most complex sensory organs. The neuroinformatics scientist says: “Science has, for a long time, failed to understand the way the sense of hearing works.”
Converting sound into a nerve impulse
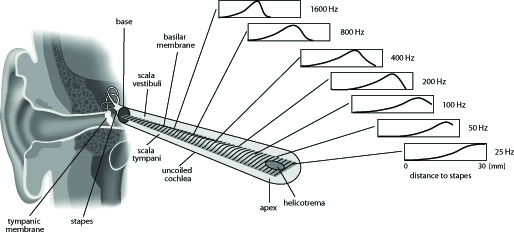
Frequencies in the form of sound waves arrive at the outer ear and pass through a canal to the eardrum, which they cause to vibrate. The tiny bones of the middle ear transmit these vibrations to the cochlea, the inner ear. There the sound waves are finally converted into a nerve impulse that is carried onwards to the brain. The various frequencies are “processed” in different regions on the cochlea; the lower notes can propagate further inward than the high ones, which fade away as early as the front-most part of the cochlea.
The hair cells located in the cochlea transform the acoustic signal into a nerve impulse which is transmitted to the brain. Various illnesses, certain medicines or exposure to very loud noises can damage the cochlea’s inner and outer hair cells, so people who are affected lose part or all of their hearing. Conventional hearing aids are scarcely able to repair such damage.
Natural hearing is theoretically possible
It is still hard to imagine that the equipment on Stoop’s office table can be shrunk to the size of a thumbnail and could one day find enough space to fit into the ear as a hearing implant. However, he thinks that this is the easier part of the job. A suitably miniaturised cochlea implant on its own is still not sufficient to restore hearing because the structure of the inner ear is very complex and its dimensions very small. Stoop says the device would need a connection through a micro cable that would have to be led directly up to the root area of the inner hair cells through a surgical operation. To do this, a surgeon would need to be able to work with micrometre accuracy.
In fact surgeons are already inserting electrodes into the inner ear nowadays for cochlea implants. To do this, they drill a channel into one of the hardest bones in the body, drill into the inner ear and place a cable into the cochlea. However, the electrode positioned in this way is rather a large distance away from the required stimulation site. This procedure would need considerable refinement to obtain the optimum situation. Stoop explains that the hearing information provided by hearing implants today is – to put it plainly – more like a mush of noise than the natural perception of sound. He says that with a new device based on their model, a patient would at last be able to hear “naturally”.
The human ear is also connected to the brain in a feedback loop through which the brain can “tell” the hair cells which signals it wants to “hear”. The neuroinformatics scientists also want to tap into this control circuit, which is not yet possible at present.
The biophysics of hearing unravelled
The neuroinformatics scientists led by Stoop developed a mathematical model to arrive at the hardware cochlea set up on his office table. Through this they succeeded in understanding the biophysics of the sense of hearing.
He says this understanding now also allows them to predict the operations that are needed to bridge over gaps in the hearing spectrum by using biophysical intervention on the basilar membrane. Most people with hearing damage no longer hear a particular range of tonal pitches because the part of the cochlea where these frequencies are detected is damaged. Through changes on the basilar membrane, a situation is reached in which these sound pitches can nevertheless be perceived in the healthy region.
Stoop says this could be achieved, for example, by altering the stiffness or mass of the basilar membrane. “We can calculate everything but we still lack the corresponding surgical methods.” The paper, which was published recently in PLoS, shows first and foremost the potential of the new method to improve the hearing of people with damaged hearing.
A neural network is needed
Another hard nut to crack on the way to a high-quality application of the electronic cochlea is to find out how the device can be controlled to turn the undifferentiated hearing of sound into selective and attentive listening. Although the cochlea can be subdivided into various regions that absorb different frequencies, these parts can also communicate with one another. Stoop says, “All the frequencies are knotted together in a complicated way. What we need for optimum control is a neural network.” The researchers are working flat out on this. They are also looking for people who want to collaborate with them to implement the new possibilities medically or technically.
Literature reference
Kern A, Heid C, Steeb WC, Stoop N, Stoop R: Biophysical Parameters Modification Could Overcome Essential Hearing Gaps. PLoS Comput Biol 4(8): e1000161.







READER COMMENTS